Real Data with Simulated Signals: Part I
Lei Sun
2017-05-18
Last updated: 2017-06-16
Code version: 41a6d4b
library(ashr)
library(edgeR)
library(limma)
library(qvalue)
library(seqgendiff)
library(sva)
library(cate)source("../code/gdash.R")Introduction
Using David’s package seqgendiff, we are adding artefactual signals to the real GTEx Liver RNA-seq data.
mat = read.csv("../data/liver.csv")The true signal comes from a mixture distribution
\[
g\left(\beta\right) = \pi_0\delta_0 + \left(1 - \pi_0\right)N\left(0, \sigma^2\right)
\] The simulated data matrices are then fed into edgeR, limma pipeline. In the following simulations, we always use \(5\) vs \(5\).
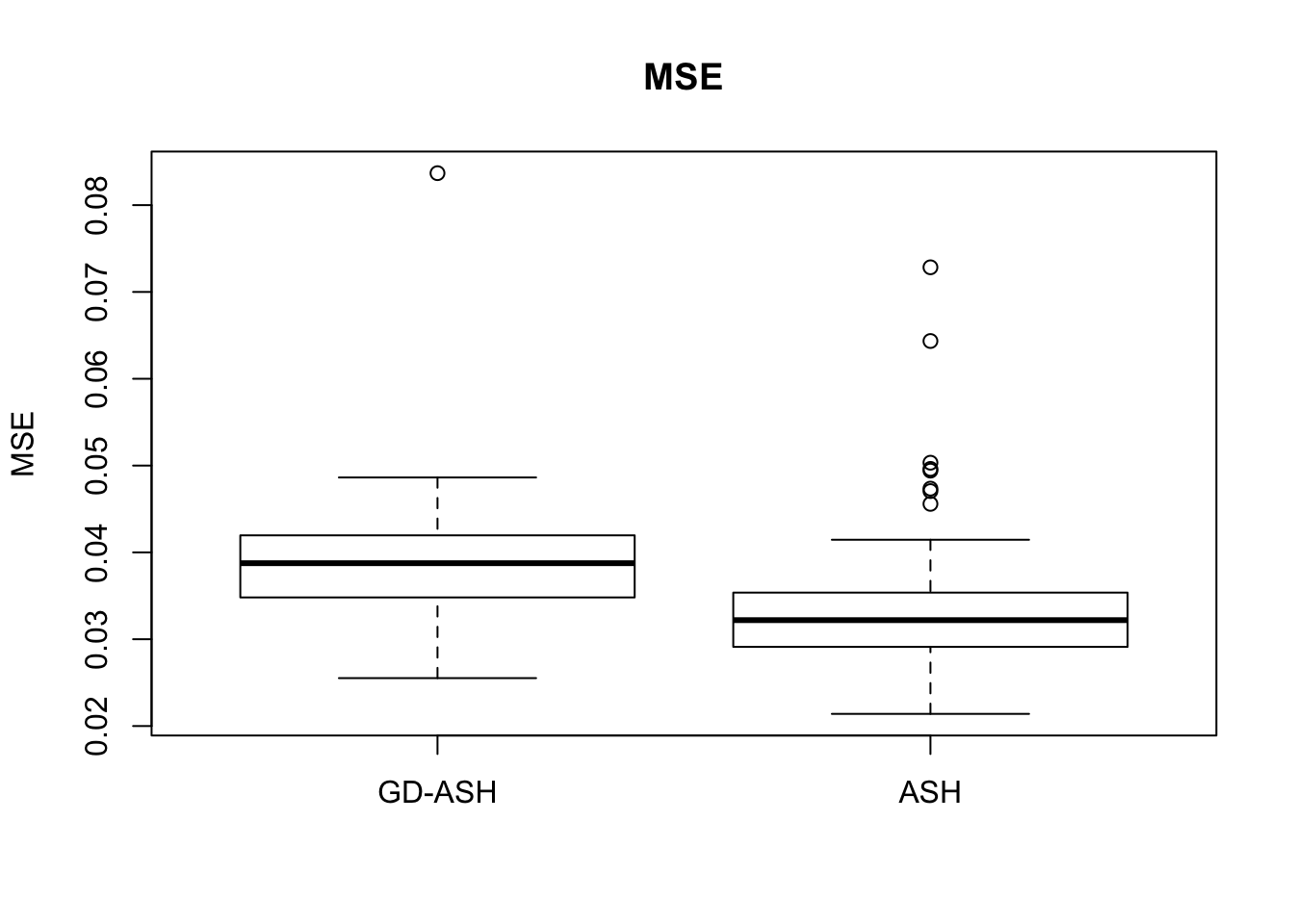
Case 1: \(\pi_0 = 0.9\), \(\sigma^2 = 1\).
N = 100
nsamp = 10
pi0 = 0.9
sd = 1
system.time(ashvgdash <- N_simulations(N, mat, nsamp, pi0, sd)) user system elapsed
6854.603 627.845 12855.081 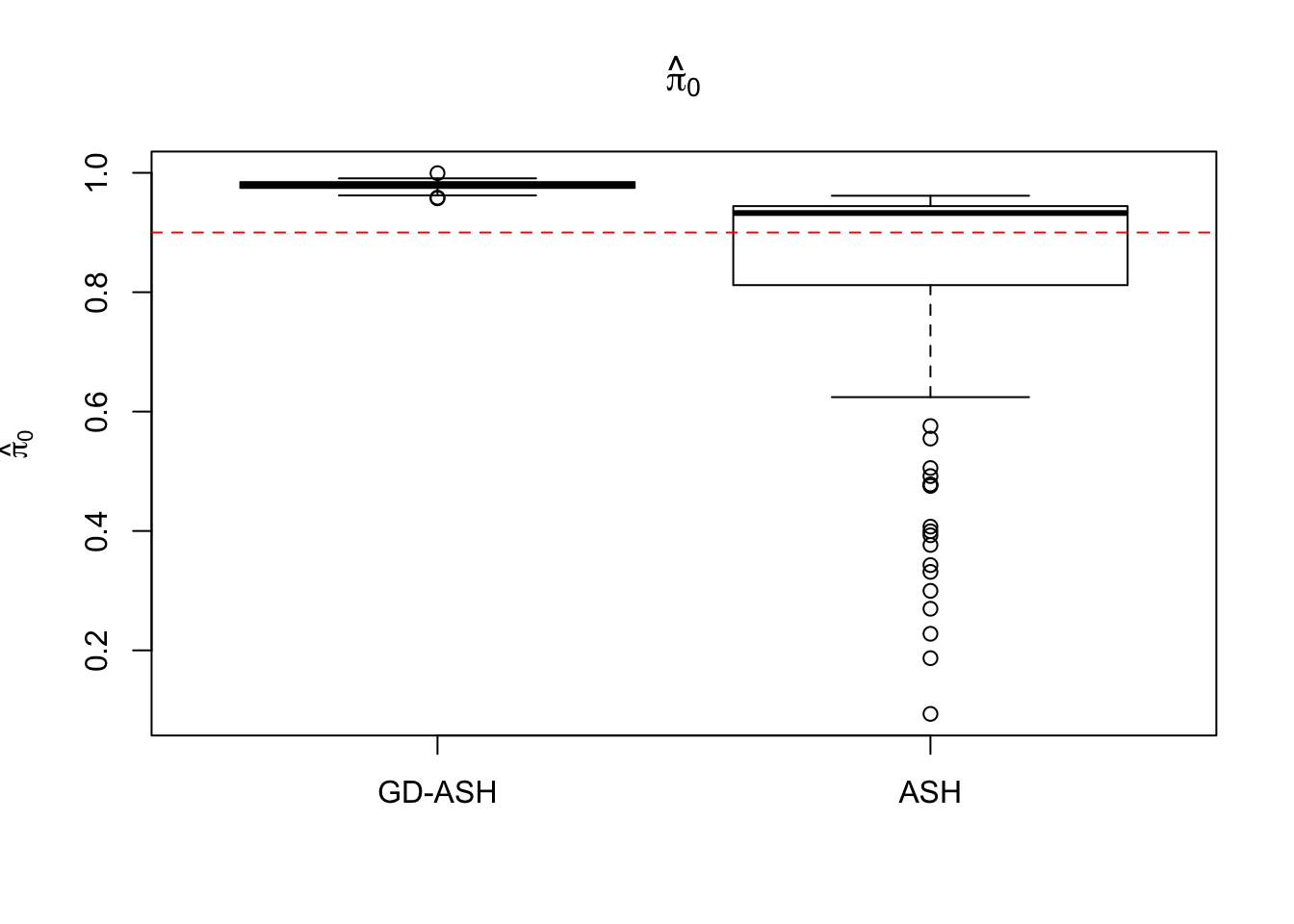
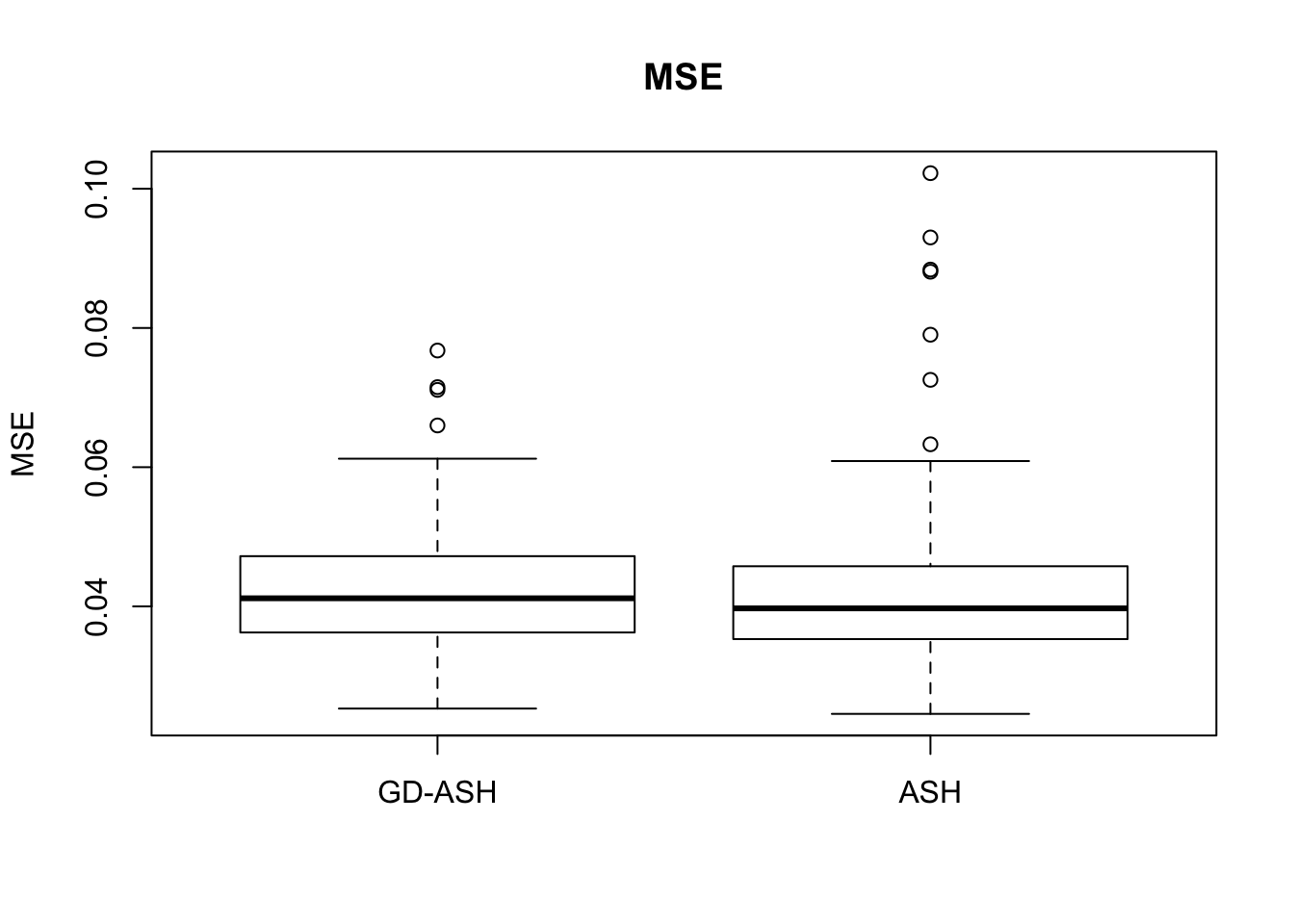
Case 2: \(\pi_0 = 0.9\), \(\sigma^2 = 4\)
N = 100
nsamp = 10
pi0 = 0.9
sd = 2
system.time(ashvgdash <- N_simulations(N, mat, nsamp, pi0, sd)) user system elapsed
5877.223 621.433 5256.693 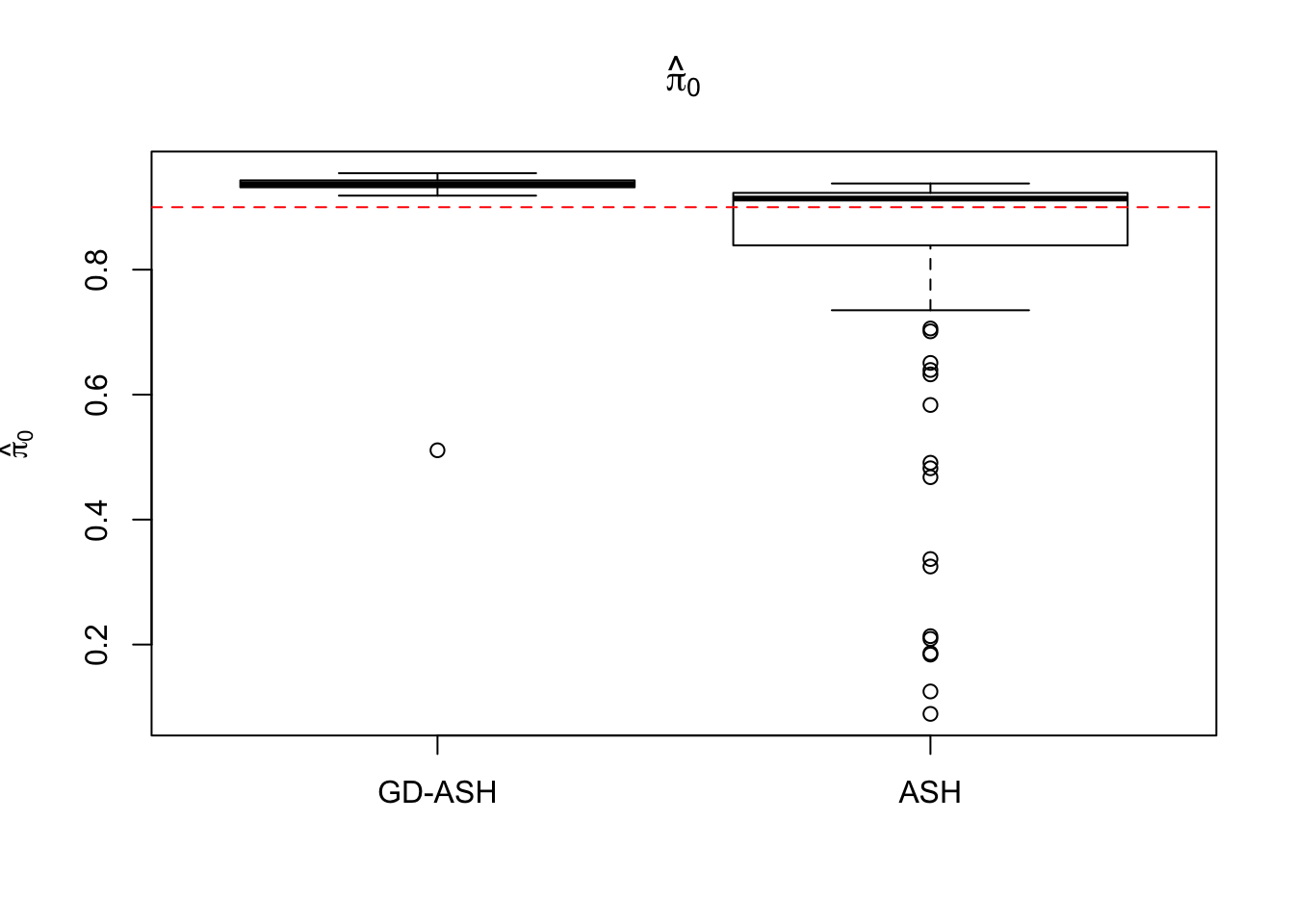
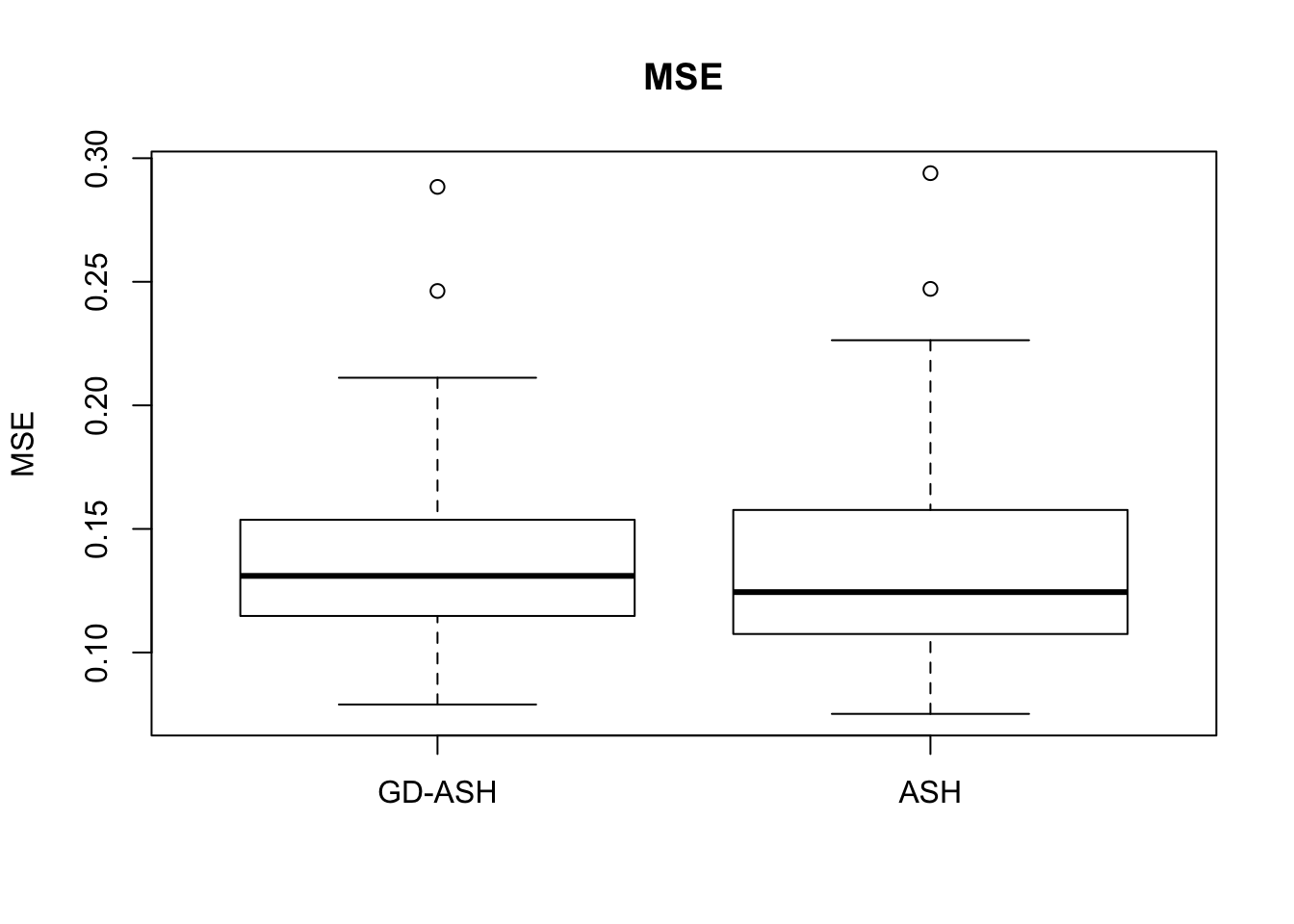
Case 3: \(\pi_0 = 0.5\), \(\sigma^2 = 4\)
N = 100
nsamp = 10
pi0 = 0.5
sd = 2
system.time(ashvgdash <- N_simulations(N, mat, nsamp, pi0, sd)) user system elapsed
5269.488 574.588 15042.479 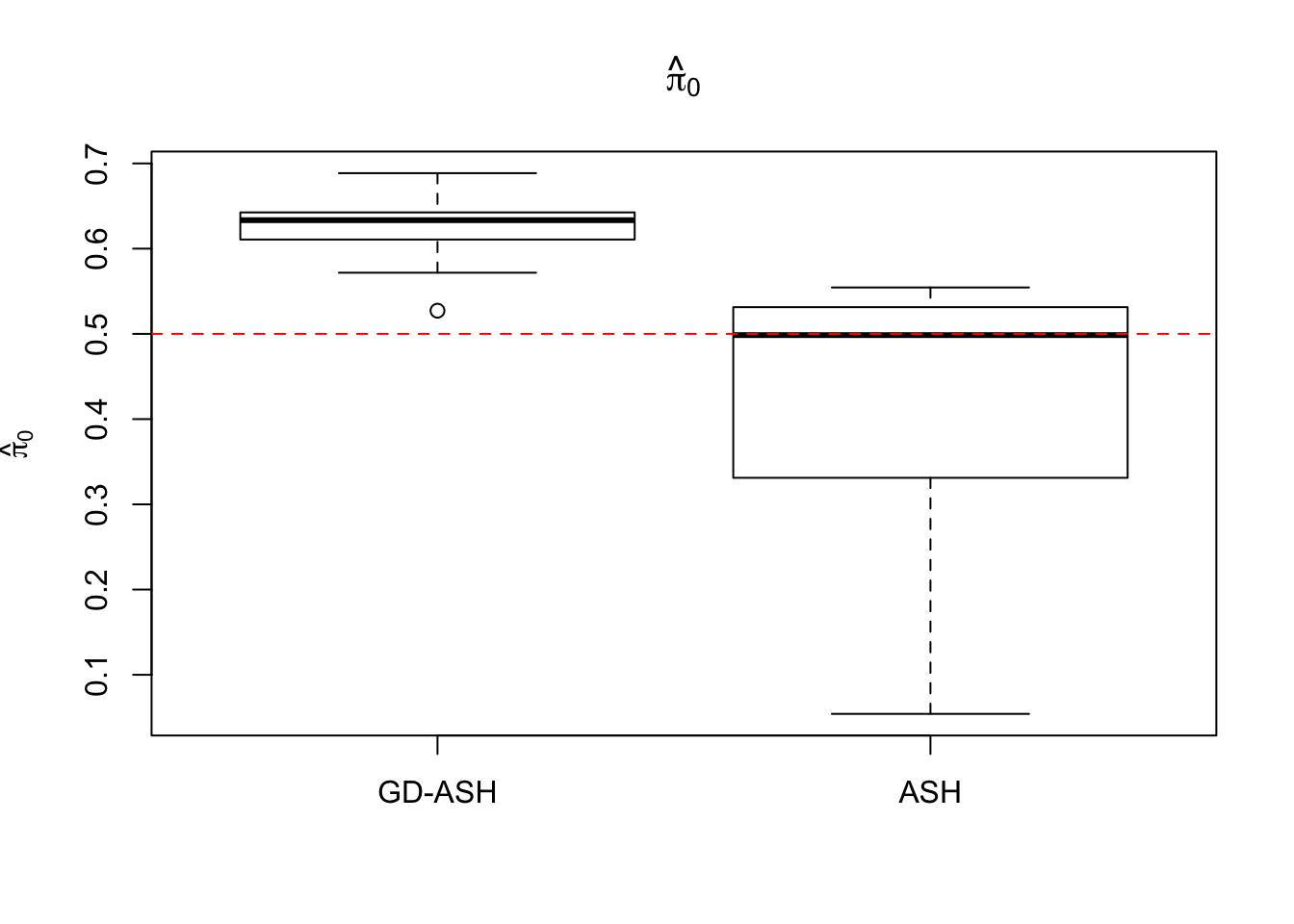
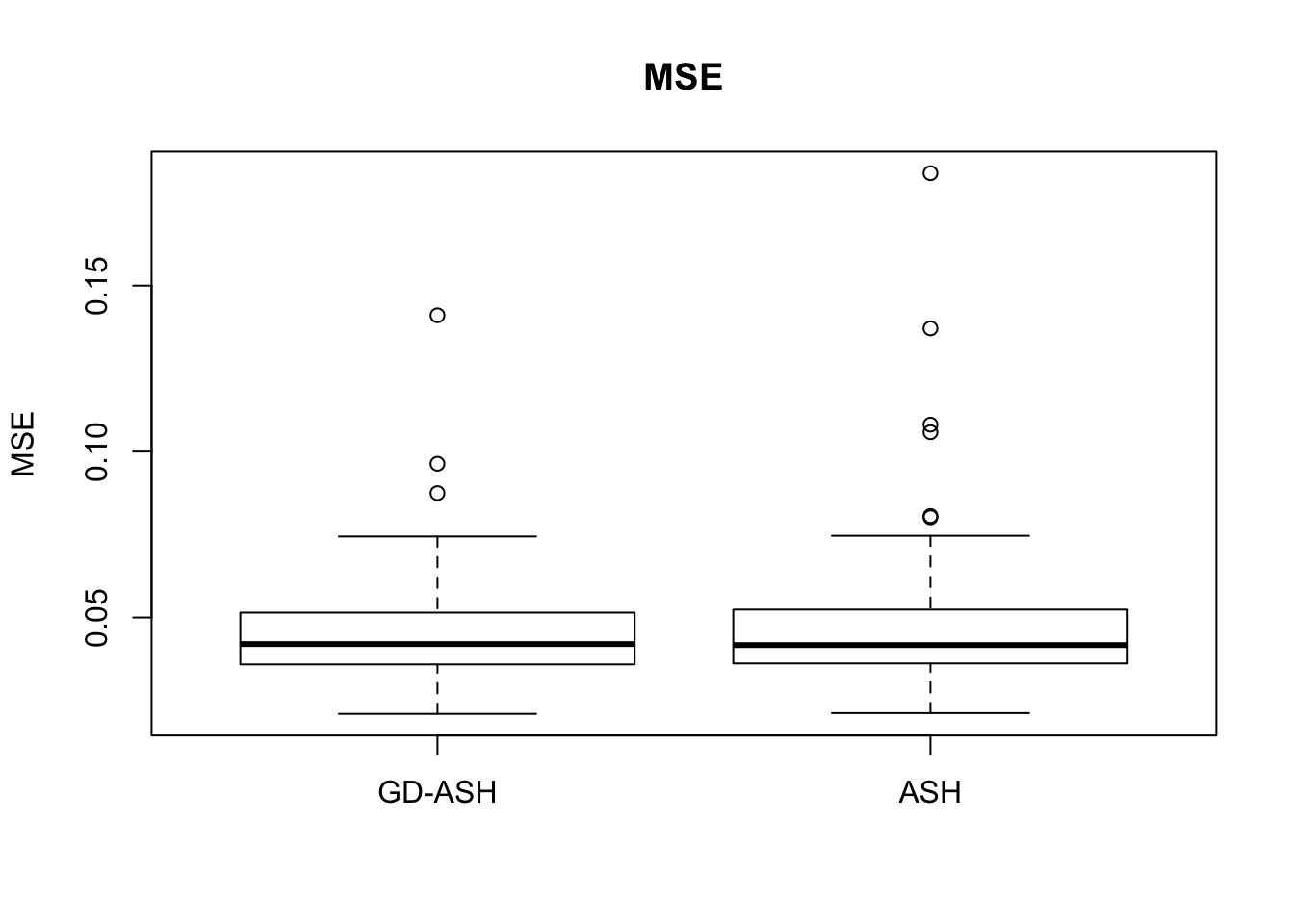
Case 4: \(\pi_0 = 0.9\), \(\sigma^2 = 9\)
N = 100
nsamp = 10
pi0 = 0.9
sd = 3
system.time(ashvgdash <- N_simulations(N, mat, nsamp, pi0, sd)) user system elapsed
5321.625 558.205 15356.345 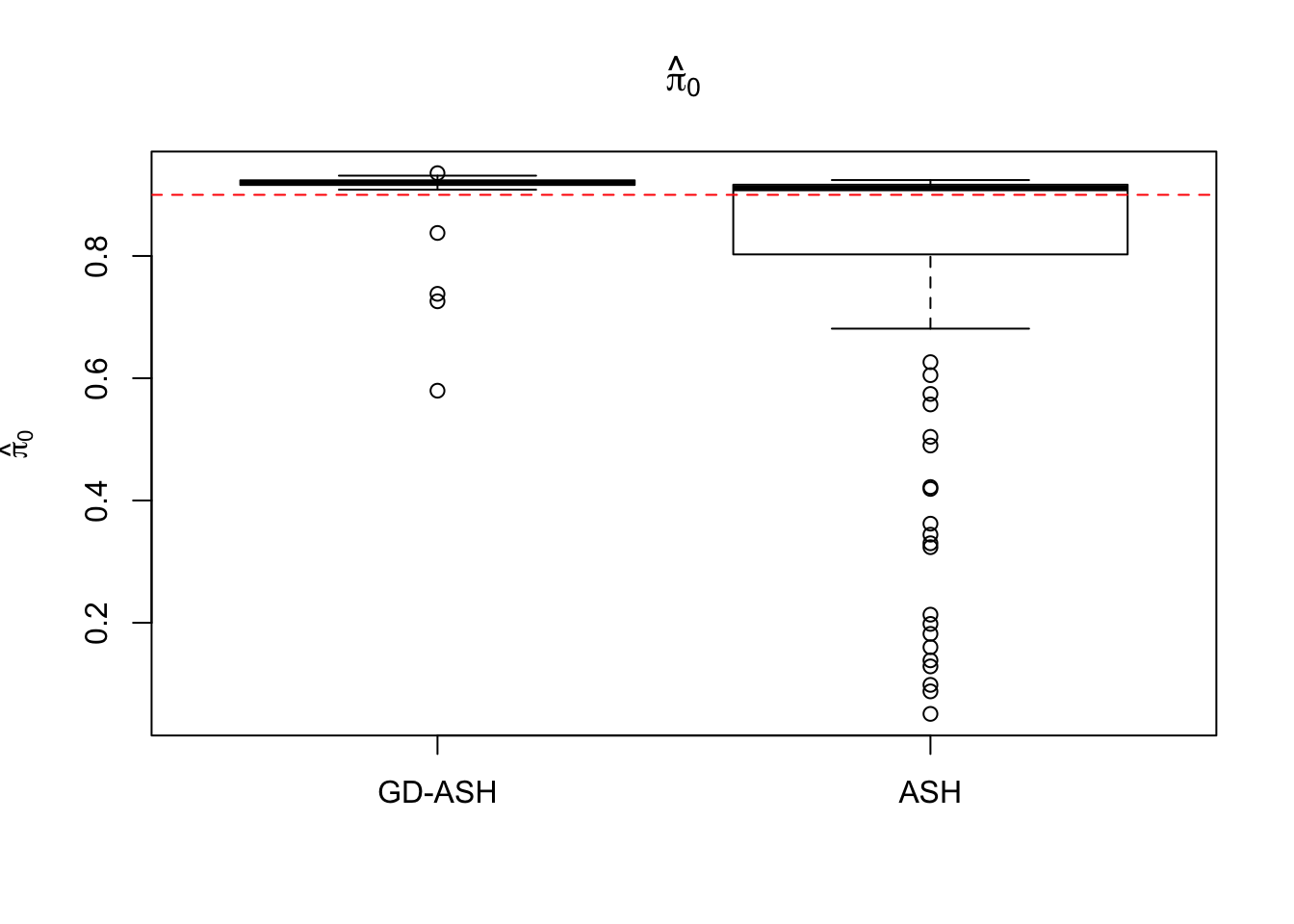
Session information
sessionInfo()R version 3.3.3 (2017-03-06)
Platform: x86_64-apple-darwin13.4.0 (64-bit)
Running under: macOS Sierra 10.12.5
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] Rmosek_7.1.2 PolynomF_0.94 cvxr_0.0.0.9009
[4] REBayes_0.85 Matrix_1.2-10 SQUAREM_2016.10-1
[7] EQL_1.0-0 ttutils_1.0-1 cate_1.0.4
[10] sva_3.20.0 genefilter_1.54.2 mgcv_1.8-17
[13] nlme_3.1-131 seqgendiff_0.1.0 qvalue_2.4.2
[16] edgeR_3.14.0 limma_3.28.21 ashr_2.1-13
loaded via a namespace (and not attached):
[1] reshape2_1.4.2 splines_3.3.3 lattice_0.20-35
[4] colorspace_1.3-2 htmltools_0.3.6 stats4_3.3.3
[7] yaml_2.1.14 XML_3.98-1.7 survival_2.41-3
[10] rlang_0.1.1 DBI_0.6-1 BiocGenerics_0.18.0
[13] foreach_1.4.3 plyr_1.8.4 stringr_1.2.0
[16] leapp_1.2 munsell_0.4.3 gtable_0.2.0
[19] svd_0.4 codetools_0.2-15 evaluate_0.10
[22] memoise_1.1.0 Biobase_2.32.0 knitr_1.16
[25] IRanges_2.6.1 doParallel_1.0.10 pscl_1.4.9
[28] parallel_3.3.3 AnnotationDbi_1.34.4 esaBcv_1.2.1
[31] Rcpp_0.12.10 corpcor_1.6.9 xtable_1.8-2
[34] scales_0.4.1 backports_1.0.5 S4Vectors_0.10.3
[37] annotate_1.50.1 truncnorm_1.0-7 ggplot2_2.2.1
[40] digest_0.6.12 stringi_1.1.5 grid_3.3.3
[43] rprojroot_1.2 tools_3.3.3 bitops_1.0-6
[46] magrittr_1.5 lazyeval_0.2.0 RCurl_1.95-4.8
[49] tibble_1.3.1 RSQLite_1.1-2 MASS_7.3-47
[52] ruv_0.9.6 rmarkdown_1.5 iterators_1.0.8
[55] git2r_0.18.0 This R Markdown site was created with workflowr